Aemcolo is indicated for the treatment of Travelers’ Diarrhea (TD) caused by noninvasive strains of Escherichia coli in adults which
is not complicated by fever and/or bloody stool.


APPROVED USE FOR AEMCOLO
Targeted Travelers’
Diarrhea (TD) Therapy
Fast, Effective Relief
For travelers’ diarrhea caused by noninvasive strains of E. coli1
Nonsystemic
<0.1% bioavailability with low
potential for clinically relevant
drug-to-drug interactions1
MMX® Technology
Designed to target treatment
at the sites of infection, the
distal small bowel and colon2-4
Certain travelers are at particular risk for Travelers’ Diarrhea
Susceptible populations include people with5:
- Weakened immune systems
- Cirrhosis of the liver
- Regular use of acid blockers or antacids
- Diabetes, inflammatory bowel disease and other chronic conditions
Advantage of nonsystemic Aemcolo treatment
Up to 45% of travelers on chronic medications are at risk for drug-to-drug interactions with travel medications like systemic antibiotics for travelers’ diarrhea.6
Systemic antibiotics carry a risk of drug-to-drug interactions (DDIs) with many common regular medications, such as blood thinners, COPD or asthma medications, muscle relaxants, antiretrovirals and even certain antacids.6
Unlike systemic antibiotics, Aemcolo has a low potential for clinically relevant drug-to-drug interactions.

Based on the minimal systemic rifamycin concentrations
observed after the recommended dose of Aemcolo,
clinically relevant DDIs are not expected.1
Fast, effective relief from Travelers’ Diarrhea
Pivotal Study*1,7

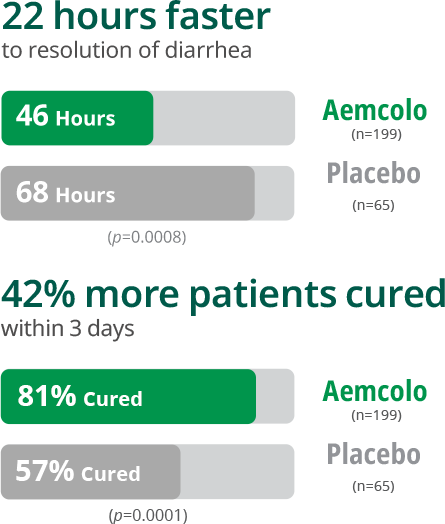
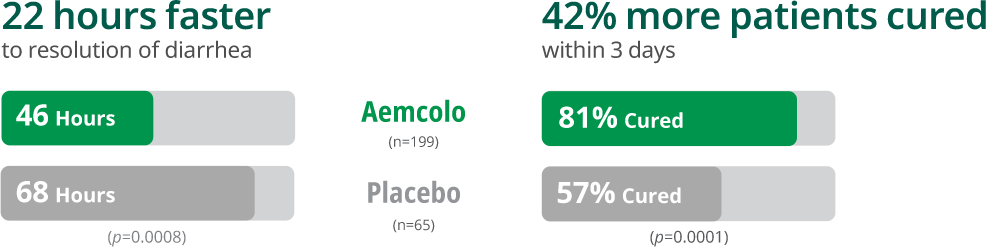
*Results from a multicenter, randomized, double-blind, placebo-controlled study of 264 adult patients. The primary efficacy endpoint was median time to last unformed stool (resolution of diarrhea) in the intent to treat (ITT) population. The clinical efficacy of Aemcolo was assessed using an endpoint of time to last unformed (watery or soft) stool (TLUS) before achieving clinical cure. The endpoint of clinical cure was defined as two or fewer soft stools and minimal enteric symptoms at the beginning of a 24-hour period or no unformed stools at the beginning of a 48-hour period.
Supportive Non-inferiority Study†1,2
Aemcolo vs. ciprofloxacin in acute Travelers’ Diarrhea
Median TLUS
resolution of diarrhea

Primary endpoint: Median time to last unformed stool (TLUS)
(p=0.0011 for non-inferiority)
†Results from a multicenter, randomized, double-blind, non-inferiority study conducted in India, Ecuador and Guatemala comparing Aemcolo (n=420) with ciprofloxacin (n=415) in 835 patients supported the superiority results found in the pivotal Aemcolo vs. placebo study. The primary efficacy endpoint was median time to last unformed stool (resolution of diarrhea) in the full analysis set (FAS) population. The clinical efficacy of Aemcolo was assessed using an endpoint of time to last unformed (watery or soft) stool (TLUS) before achieving clinical cure. The endpoint of clinical cure was defined as two or fewer soft stools and minimal enteric symptoms at the beginning of a 24-hour period or no unformed stools at the beginning of a 48-hour period.
CDC and ISTM guidelines suggest early intervention with an antibiotic may reduce symptoms and duration of TD.8,9
Delayed-release MMX® technology for targeted medication delivery
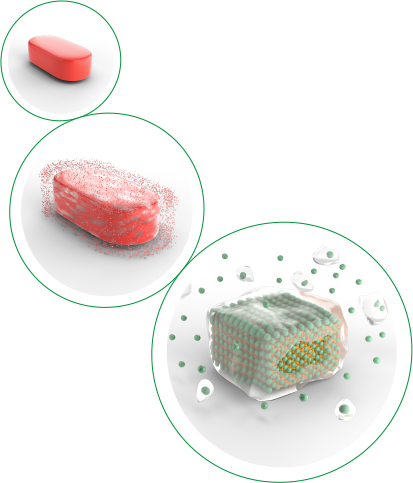
MMX® technology uses a two-part delayed
release system2-4:
1. pH-resistant film keeps the tablet intact until it reaches the distal small bowel
2. Viscous gel vehicle extends release of rifamycin throughout the distal small bowel and colon
Delivers medication directly
to the sites of infection2-4:
• Bypasses upper GI tract exposure; low risk of adverse events and DDIs
• Delivers a high concentration of rifamycin directly to the sites of infection, the distal small bowel and colon
Before your patients on chronic medications travel,
prescribe Aemcolo instead of systemic antibiotics for TD.
Reliable safety and tolerability
Most common adverse reactions
incidence ≥ 2%
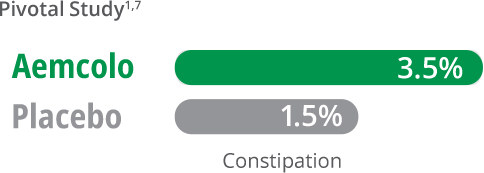
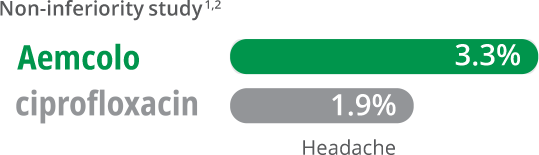
- In two clinical trials with 619 adults, the most common adverse reactions in Aemcolo patients (incidence ≥ 2%) were Study One: constipation (3.5%) and Study Two: headache (3.3%)
- Discontinuation due to adverse reactions occurred in 1% of patients
- The most frequent causes of discontinuations in Aemcolo patients were abdominal pain (0.5%) and pyrexia (0.3%)
Simple Dosing
2 tablets

Twice Daily

For 3 days

Dosing instructions
- Aemcolo may be taken with or without food. Do NOT take Aemcolo concomitantly with alcohol
- Tablets should be swallowed whole with a full glass of liquid and not crushed, chewed or broken
Counsel patients that it is important to take the antibiotic exactly as directed. Skipping doses or ending the medication early may lower how well the drug will work and may increase the chance of the bacteria becoming resistant.
Travelers’ diarrhea risk around the globe
Prepare your patients for travel
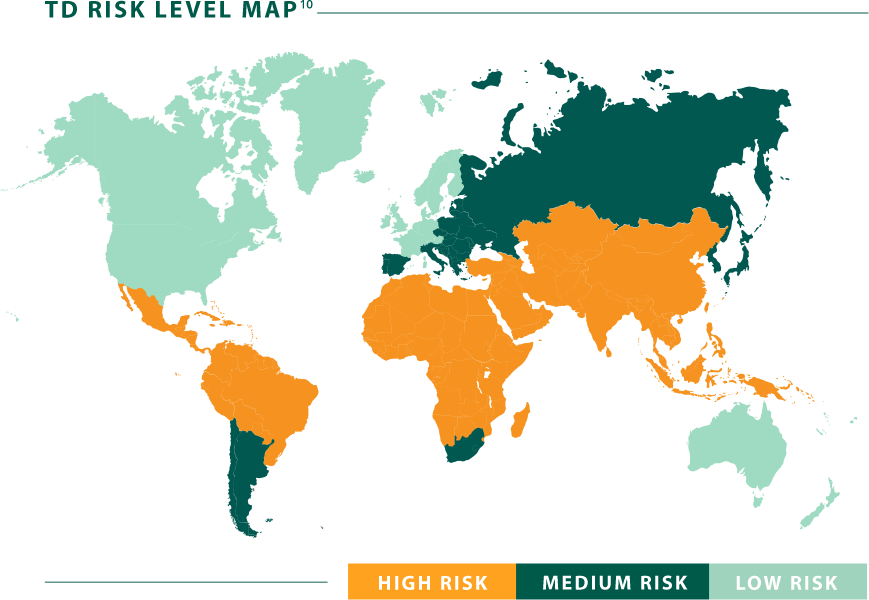
Before patients travel, prescribe Aemcolo.
REFERENCES:
1. Aemcolo [package insert]. Raleigh, NC: Redhill Biopharma; 2019. 2. Steffen R, Jiang ZD, Gracias Garcia ML, et al. Rifamycin SV-MMX® for treatment of traveler’s diarrhea: equally effective as ciprofloxacin and not associated with the acquisition of multi-drug resistant bacteria. J Travel Med. 2018;25(1):1-11. https://doi.org/10.1093/jtm/tay116. 3. Di Stefano AFD, Rusca A, Loprete L, Dröge MJ, Moro L, Assandri A. Systemic absorption of rifamycin SV MMX® administered as modified-release tablets in healthy volunteers. Antimicrob Agents Chemother. 2011;55(5):2122-2128. 4. Nardelli S, Pisani L, Tontini G, et al. MMX® technology and its applications in gastrointestinal diseases. Ther Adv Gastroenterol. 2017;10(7):545-552. 5. Mayo Clinic Staff. Travelers’ Diarrhea. https://www.mayoclinic.org/diseases-conditions/travelers-diarrhea/symptoms-causes/syc-20352182, Accessed Apr 3, 2020. 6. Youngster I, Barnett ED. Centers for Disease Control and Prevention. Interactions between Travel Vaccines & Drugs. https://wwwnc.cdc.gov/travel/yellowbook/2020/preparing-international-travelers/interactions-between-travel-vaccines-and-drugs, Accessed Nov 3, 2020. 7. DuPont, HL, et al. Targeting of Rifamycin SV to the Colon for Treatment of Travelers’ Diarrhea: A Randomized, Double-Blind, Placebo-Controlled 3 Study. J Travel Med, Vol. 21:369–376, 2014. 8. Riddle, M, et al. Guidelines for the prevention and treatment of travelers’ diarrhea: a graded expert panel report. J Travel Med, Vol. 24(1): S63-S80, 2017. 9. Riddle MS, Conner BA. Centers for Disease Control and Prevention. Perspective: antibiotics in travelers’ diarrhea—balancing the risks and benefits. https://wwwnc.cdc.gov/travel/yellowbook/2020/preparing-international-travelers/perspectives-antibiotics-in-travelers-diarrhea-balancing-the-risks-and-benefits, Accessed Nov 3, 2020. 10. Steffen R, Hill D, DuPont HL. Traveler’s Diarrhea: a clinical review. J Am Med Assoc. January 2015 313(1):71-80.